Morgan C. Hill, PhD, ISMPP CMPP™, Infusion, Ashfield Healthcare Communications, part of UDG Healthcare, Middletown, CT, USA; Kenneth Pomerantz, PhD, Alexion Pharmaceuticals, Inc., New Haven, CT, USA; Michael Mandola, PhD, ISMPP CMPP™, Bristol-Myers Squibb, Princeton, NJ, USA; Rebecca A. Rozich, PhD, ISMPP CMPP™, Stem Scientific, Ashfield Healthcare Communications, part of UDG Healthcare, Lyndhurst, NJ, USA; Brian Scheckner, PharmD, BCPP, ISMPP CMPP™, Jazz Pharmaceuticals, Philadelphia, PA, USA
This article is based on workshops presented at the 10th, 11th, 12th, and 13th Annual Meetings of ISMPP.
In the context of company-sponsored medical research, Good Publication Practice guidelines (GPP3) recommend the use of publication steering committees (PSCs) to “plan and oversee the development of publications and presentations from a study or group of studies.”1 A PSC is initiated by a sponsor company, may be a subgroup of a trial steering committee, and tends to function most efficiently with a limited number of participants. The goal of PSCs is to lead to increased transparency and data sharing, consistent with data disclosure and sharing initiatives, through shared collaboration between a sponsor and external experts.
A PSC can serve any of the following roles:
- Commit to publishing primary and key secondary results of a clinical trial in an objective and timely manner;
- Provide input into an initial publication plan for individual studies and participate in authorship decisions;
- Identify and provide input for publication planning of subanalyses or exploratory endpoints, based on robust medical hypotheses, that would be of interest to the scientific/medical community; and/or
- Provide input into discussions of educational needs in the therapeutic area.
A qualitative survey of ISMPP members was conducted in 20132 and repeated in 20163 to explore longitudinal differences in PSC utilization and to identify critical success factors and challenges related to the establishment of PSCs since the publication of the GPP3 guidelines.1 Of the 1284 and ~1400 surveys distributed in 2013 and 2016, respectively, responses were received from 146 (11.4%) and 117 (~8.5%) ISMPP members, respectively.3
The distribution of respondents was similar between 2013 and 2016, with approximately 40% having had prior/present agency experience, approximately 25% having had prior/present pharmaceutical company experience, and the remainder (~30–35%) having had both; for both the 2013 and 2016 surveys, more than half of respondents reported having >10 years of medical publications experience.
Since 2013, 40% of survey participants reported increased utilization of PSCs in 2016, 22% reported decreased utilization, and 38% reported no change. Only 14% of respondents reported an impact of GPP3 on PSC use; the remainder reported no impact of GPP3, but 50% did indicate that GPP3 may have a possible future impact on their PSC use.
Workshops at the ISMPP Annual Meetings provided interactive, advanced training to understand the purpose, benefit, and role of PSCs in publication planning and execution. The workshops also shared key tactics for establishing and conducting successful PSCs, including identifying critical success factors, recognizing and overcoming obstacles, and providing potential solutions to ensure PSC success.
Real-World PSC Experience
Pharmaceutical Perspective
Per GPP3 guidelines, PSC best practices include formation prior to data availability to ensure sufficient discussion and planning of resultant publication(s) and involvement of study investigators, sponsor employees (eg, scientists, clinicians, or statisticians), contractors involved in the study, therapeutic area experts, or publication professionals.1 Most commonly, PSCs consist of company scientists and external investigators who may have contributed to clinical protocol design or trial development, and serve a leadership role in the conception, development, and execution of a study, analysis, and/or publication plan.
Results from the 20132 and 20163 surveys suggested a trend toward more PSCs derived from phase 3 clinical programs (in which 64% and 74% of respondents participated, respectively) and single phase 3 studies (34% and 48%, respectively), while PSCs derived from phase 2 clinical programs (21% and 17%, respectively) and single phase 2 studies (15% and 13%, respectively) appeared to be trending downward.
Specific composition of PSCs and corresponding logistics, however, may vary depending on the clinical development program or clinical study. As a result, questions regarding these issues are common from publication professionals. Table 1 below presents key questions regarding PSC composition and logistics posed by survey respondents and summarizes survey results3 and authors’ observations regarding each issue.
Table 1. Observations and trends regarding PSC logistics

Selection of external PSC members can also involve factors beyond those presented in Table 1. For instance, selection of one investigator over another for invitation to join a PSC based on numbers of publications in the therapeutic area may provide a proxy measure of therapeutic expertise and, potentially, familiarity with the publication process. In contrast, selection of one investigator over another based on their enrollment of patients into a clinical trial may be less helpful. In the case of rare diseases, higher relative patient enrollment may indicate increased clinical experience; however, as with authorship criteria,1 simply enrolling the most patients in a given trial may not be justification on its own for inclusion in a PSC.
Once the membership of a PSC has been established, the logistics of live versus virtual meetings may also need to be considered. Live meetings, while conducive to extensive discussions and ideal for longer meetings, also often involve coordination of multiple busy schedules; potential medical, legal, regulatory, and/or compliance reviews; and payment/compensation/budgetary issues (separate from payment for authorship considerations) that may have ramifications on Sunshine Act reporting. Conversely, teleconferences, while not conducive to discussions longer than ~2 hours and lacking live interactions, are easier to schedule, may not require the same rigor of medical/legal/regulatory/compliance oversight, often require no associated payment, and can be supplemented with virtual technology (eg, WebEx, Skype).
Agency Perspective
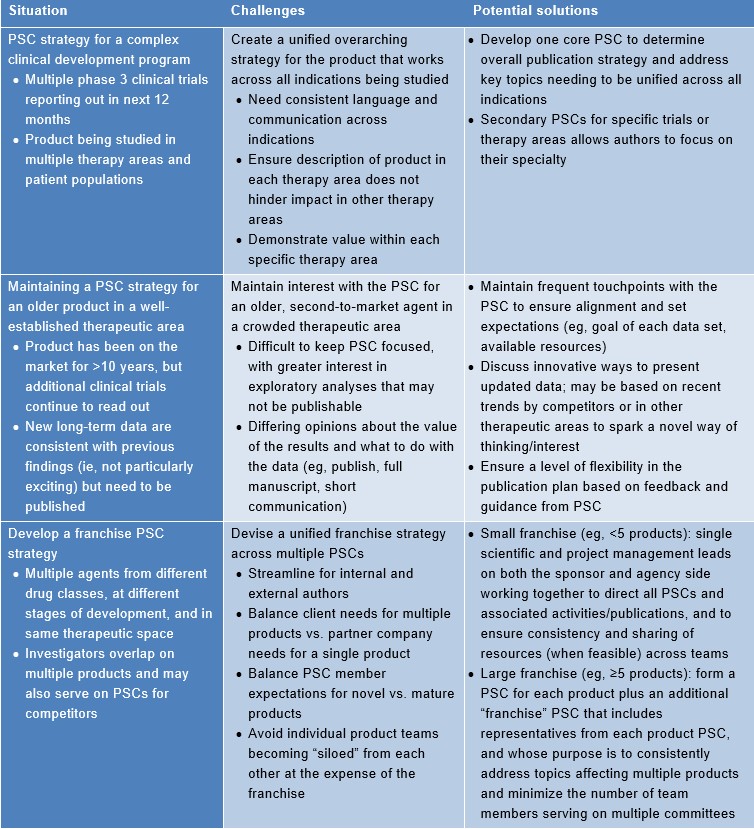
Agencies can play a key supportive role in PSC development, logistics, and strategy. An agency can assist a sponsor with identification and invitation of appropriate PSC members, ensure consistency with best publication practices, organize all aspects (eg, logistics, agenda, moderation) of PSC meetings, and maximize author participation in PSC meetings via logistical solutions and/or innovations. Furthermore, an agency is well positioned to translate PSC feedback into publications via medical writing support, and to engage relevant parties promptly to address gaps in data/research identified by PSC members. Table 2 below summarizes several complex PSC challenges and associated potential solutions from an agency perspective.
Table 2. Agency perspective on complex PSCs: associated challenges and potential solutions
Critical Success Factors
Critical success factors associated with successful PSCs, including key attributes identified by survey respondents, are described in Table 3 below. While 2013 survey respondents indicated that the most important aspects of a successful PSC included “engagement” and “roles/responsibilities,” the relative importance of “driving to consensus” increased among 2016 survey respondents.2,3 In both the 2013 and 2016 surveys (Figure 1 below), the topics of “selection process for PSC membership,” “internal policies,” and “agreement to charter principles” were identified as key hurdles/challenges to PSC development and execution.2,3
Table 3. Critical attributes of a successful PSC from survey respondents2,3

Figure 1. Significance of hurdles/challenges associated with developing and executing a PSC as assessed by 2016 survey respondents2,3

CIA=corporate integrity agreement; COI=conflict(s) of interest; HCP=health care practitioner
Results from both ISMPP member surveys highlight the importance and incremental value of PSC charters, agreements, or other guidance documents whose overall purpose is to provide a road map for the development and execution of a publication plan.2,3 Specifically, PSC charters define roles/responsibilities of their members1 and ensure adherence to publication policies/transparency. Typically, the initial draft of a PSC charter is generated by a sponsor, reviewed/customized by all PSC members, reviewed/approved by the sponsor’s legal/compliance team, then signed by all PSC members. While a PSC agreement is similar to a PSC charter, it may be shorter, is not typically discussed unless questions arise, and may be distributed for signature in advance of the initial PSC meeting.
Evolution of PSCs
Since their inception in 2003, the GPP guidelines4 have acknowledged the fundamental importance of cooperation and communication between internal (ie, company-based) and external (ie, academic or clinical) stakeholders in the publication of company-sponsored medical research. In the 2009 GPP2 update,5 this need was addressed by the introduction of the concept of a PSC, the roles and responsibilities of which were further refined in the 2015 GPP3 update.1 However, given the complexities inherent in company-sponsored medical research, the establishment and utilization of successful PSCs have encountered certain challenges and hurdles. This led the authors to conduct a survey of publication professionals to better understand how PSCs are utilized and how they may have been impacted by the evolution of the GPP guidelines.
Results from the 2013 and 2016 surveys2,3 confirmed that PSC utilization is increasing over time and that publication professionals are contributing to the evolution of PSCs to ensure effective communication, cooperation, and transparency. Ongoing discussions of PSC logistics, challenges, trends, and best practices, including this workshop that was presented at each ISMPP Annual Meeting for the past four years, have provided continuing guidance and clarity to drive PSC success. Although the impact specifically of GPP3 on PSC utilization was minimal as of the 2016 ISMPP survey, many survey respondents at the time anticipated a possible future impact, and discussions from the 2017 workshop were consistent with that aspiration.
Disclaimer
The ideas and opinions presented and discussed in this article are solely those of the individual authors and should under no circumstances or in any way be considered to reflect those of their employers or any organizations of which they may be members.
References
- Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015; 163: 461-464.
- Scheckner B, Pomerantz K, D’Angelo G, et al. A combined qualitative/quantitative survey assessment of publication steering committee usage patterns and operationalization. Curr Med Res Opin 2014; 30: S9.
- Scheckner B, Pomerantz K and Mandola M. The evolving use of publication steering committees (PSCs). Curr Med Res Opin 2017; 33: 10-11.
- Wager E, Field EA and Grossman L. Good publication practice for pharmaceutical companies. Curr Med Res Opin 2003; 19: 149-154.
- Graf C, Battisti WP, Bridges D, et al. Research methods & reporting. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ 2009; 339: b4330.

